Get PolitiFact in your inbox.

(Shutterstock)
If Your Time is short
-
Federal regulators have sent more than 180 warning letters to businesses that have violated federal law by claiming their products can treat or prevent the coronavirus.
-
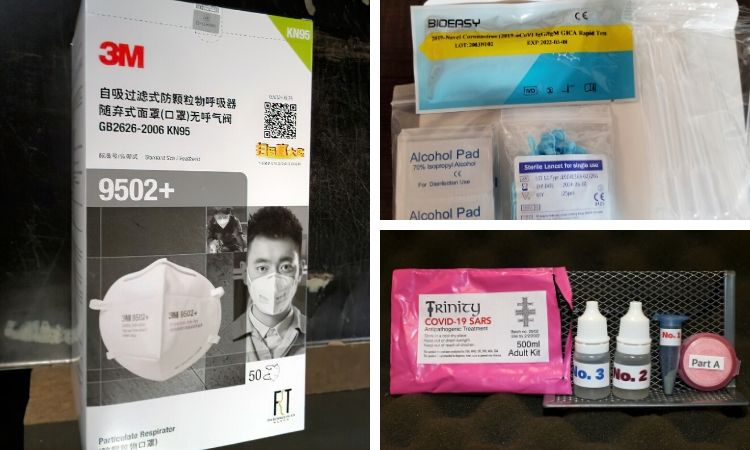
Bogus coronavirus treatment products include copper “hand sanitizer” and IV machines.
-
These businesses got a significant bump in their engagement with social media users in March, as COVID-19 cases began to climb and the country started shutting down.
Scott Zalkind was just trying to keep his one-man business safe.
As the owner of Lucky Dog Hot Sauce in Hayward, Calif., Zalkind relies on farmers markets to make sales. So when the markets started to require face masks, he started shopping around online. With the nationwide shortage from COVID-19, it took a while before he found some that were described as "N95 filter masks." They were being sold by a Chinese company he had never heard of.
"I found this one site and I thought, ‘It’s double the price but screw it — I need them,’" Zalkind said.
Zalkind purchased a pack of five — the masks cost $10 each plus a $10 shipping fee — and they took three weeks to arrive. The package was thinner than he expected for a set of bulky N95s, which filter out at least 95% of airborne particles.
When he opened it, Zalkind saw that the masks were labeled as KN95s. They had no filter. He complained to the company, but it sent him only a partial refund.
"It’s a straight bait-and-switch," he said. "I can’t be the only one they did this to."
He isn’t. Americans have reported more than 32,000 cases of coronavirus-related fraud, according to the Federal Trade Commission. The agency estimates that consumers have lost $3.6 million in online shopping alone, though it’s hard to say with certainty since not everyone files a complaint when they’ve been scammed.
The onslaught of fraud complaints has left federal and state regulators scrambling to stop scammers looking to cash in on the coronavirus outbreak. But with no end to the pandemic in sight, and businesses exploiting social media platforms like Facebook to sell their bogus products, hundreds of thousands of Americans run the risk of being ripped off.
In this March 19, 2020 photo, a shopper looks for toilet paper at a Stop & Shop supermarket during hours open daily only for seniors in North Providence, R.I. (AP)
The breadth of fraudulent products is "amazing," said Rich Cleland, assistant director of advertising practices at the federal Bureau of Consumer Protection. And it’s not just claims of undelivered or unusable personal protective equipment.
The agency is flooded by reports of products that falsely claim to prevent or treat COVID-19. The absence of an effective treatment for COVID-19 tempts worried consumers to take a chance on sketchy companies they see on their news feeds or Google searches. (To prevent infection, the Centers for Disease Control and Prevention advises people to wash their hands regularly, avoid touching their face and wear masks in public.)
It’s against federal law to sell a drug that’s unapproved or unproven to treat a certain illness, according to the Federal Food, Drug, and Cosmetic Act. It’s also illegal to misbrand a drug and sell it. The Food and Drug Administration enforces the law in partnership with the FTC, which regulates deceptive business practices.
Since March, the FTC has sent more than 180 warning letters to companies, individuals and organizations that allegedly violated federal law by falsely claiming their products can treat or prevent COVID-19. The FDA also has sent scores of warnings to firms selling bogus products. The offending businesses range from herb companies in California to chiropractors in Georgia.
"The numbers of warning letters that we have sent out are really unprecedented," Cleland said. "I’ve worked through various public health scares before, like SARS and Ebola. We sent out warning letters in those cases, and the numbers of warning letters all combined don’t equal the number that we’ve sent out related to COVID-19."
States hard-hit by the pandemic, such as Michigan and New York, have taken similar steps to contain coronavirus-related scams. Just at the state level, there are hundreds of consumer complaints to sift through.
"We usually average about 80 calls" per day, Joe Potchen, chief of the Michigan Consumer Protection Bureau, said in early May. "Now we’re averaging over 250 calls a day."
Consumer protection agencies are used to dealing with rogue operators scamming people for money. But coronavirus-related scams have the potential to cause more harm than a standard financial scam. Fraudulent testing kits and substandard face masks could give people a false sense of security, regulators fear, while misused products like bleach or silver solution could land them in the hospital.
"You’re really talking about a situation in which you might be killing people," said Michigan Attorney General Dana Nessel.
On a Feb. 12 broadcast of his talk show, televangelist Jim Bakker held up a blue and silver bottle in front of his guest, "naturopathic doctor" Sherrill Sellman.
"This influenza that is now circling the globe," Bakker said, incorrectly describing the coronavirus as a flu. "You're saying that Silver Solution would be effective."
Sellman responded: "Well, let's say it hasn't been tested on this strain of the coronavirus, but it has been tested on other strains of the coronavirus and has been able to eliminate it within 12 hours."
A message on the screen said 4-ounce bottles of Silver Solution could be yours for $80. There was just one problem — the product isn’t proven to treat any illness, including COVID-19.
According to the FTC, Bakker, who’s based in Missouri, violated federal law by promoting a bogus medical cure. On March 10, the state of Missouri sued him for it, after which Silver Solution was removed from Bakker’s online store.
The Bakker example was just one of several prominent coronavirus-related scams circulating in March, when searches for purported COVID-19 cures started to spike.
Around the same time, wellness influencers on Instagram started promoting supplements as ways to prevent the coronavirus. Social media accounts tried selling fake at-home testing kits. Some scammers in Los Angeles even went door to door hawking protective equipment in an attempt to get personal information of Medicare beneficiaries.
The flood of scam reports hasn’t slowed down since those early weeks.
"Every day I seem to start out further behind than I was the day before," Cleland said.
On the website for Consumer Reports, a nonprofit consumer protection organization, there are dozens of stories like Zalkind’s with the masks. Some saw ads that claimed ultraviolet light, copper and charcoal could help treat or prevent the virus. Others bought face masks online, but never received them.
"I got hosed by fullerspace.com trying to buy masks. I think I found them from a Facebook or Instagram ad," Eric Weir, an Illinois resident who said he lost about $53, told PolitiFact. "After my masks didn't ship, and didn't ship, and didn't ship, I finally Googled them and found all of the reviews that said they were a scam and never actually ship product."
Public-domain records show Fullerspace.com was registered in late March. It has dozens of one-star reviews on SiteJabber, which lets users rate online businesses to avoid falling for scams. Other scam websites have used keywords like "coronavirus" and "covid" in URLs to try to attract more visitors.
"Many of these are small, at-home businesses. Some are larger clinics," Cleland said. "They go online and they read this stuff and they believe what they see, and they pass that on to consumers to try to promote the sale of their product."
Whether scammers believe their products work, the motivation is consistent: turn a profit. "It doesn’t matter whether they’re selling toasters or CBD vaccines or masks that they’re never going to deliver," said Cleland.
(From top right) Unapproved COVID-19 tests seized at JFK Airport in New York; A phony coronavirus cure that a British man tried to smuggle into the United States; Counterfeit 3M masks confiscated at the Cincinnati LUK airport in Cincinnati. (AP)
The FTC works with state agencies to send warnings and injunctions to companies that make false claims about their products.
The FTC’s warning letters don’t have the force of a legal injunction, but Cleland said they are frequently effective in getting businesses to remove false or misleading marketing language about the coronavirus.
Linda Pavek received hers in an April 22 email. Within an hour, she complied.
"I was stunned," said Pavek, the owner of CopperTouch, a Minnesota company that sells "antimicrobial copper." "I immediately contacted my two science advisers, and we contacted our website manager to take down anything on our website that pertained to the terminology of coronavirus or COVID."
PolitiFact reached out to dozens of the businesses to which the FTC and FDA issued joint warnings. We heard back from four of them.
While each business removed language from its website about treating or preventing the coronavirus, all of them said they stood by their products. Some sent us research articles to try to substantiate their claims.
RELATED: Fact-checking COVID-19 prevention, treatment myths
But not everyone heeds the warning. And those that don’t can expect a follow-up from the FTC.
On April 27, the FTC filed a preliminary injunction barring Marc Ching, the owner of Los Angeles-based Whole Leaf Organics, from claiming that one of the company’s supplements could prevent or treat COVID-19. The product consists mainly of "vitamin C and herbal extracts," according to the FTC.
"There’s no proof that any product will prevent or treat COVID-19 or that any CBD product will treat cancer," Andrew Smith, director of the Bureau of Consumer Protection, said in a statement. "Let’s be clear: Companies making these claims can look forward to an FTC lawsuit like this one."
Ching agreed to comply with the injunction. PolitiFact reached out to him for comment, but we haven’t heard back.
In nearly all of the FTC warning letters that PolitiFact analyzed, social media accounts were listed as one of the primary ways the agency found false treatment claims.
Social-media platforms like Facebook and Instagram have adopted policies to reduce the spread of misinformation about coronavirus treatment and prevention. But they are still a key part of how scammers peddle their bogus products.
Using CrowdTangle, a tool that measures social media engagement, PolitiFact tracked the Facebook and Instagram reach of each business to which the FTC has sent warning letters. Our analysis suggests that the businesses got a big boost from the platforms in March — and they’re still reaching hundreds of thousands of people per month.
Between March 1 and 8 — the week that the FTC started to send its warning letters and the U.S. approved widespread testing for the first time — businesses that falsely claimed their products could treat or prevent COVID-19 saw their Facebook likes, shares and comments more than double. Their videos received more than six times as many views as in the previous week.
On Instagram — where alternative health stores have large followings — views, likes and comments quadrupled.
The posts, many of which were deleted after they were cited in the FTC’s letters, give a sense of how businesses used their social media accounts to falsely market their products as coronavirus treatments.
-
"Can CBD help with Corona Virus? Possibly!" said a California company called CBD Online Store in a March 9 Facebook post.
-
"Constituents in herbs exhibit a diverse array of anti-viral, virostatic, immune enhancing and anti-influenza activities that may prevent and treat pandemic influenza," said Irish company Carahealth in another post.
-
"ESSENTIAL OIL RESPIRATORY FORMULA – Soothing Support for Symptoms like Corona Virus," promised a March Facebook post from a website called Pure Plant Essentials.
As the FTC started sending more letters, the businesses’ social media engagement dropped, hitting a low point in mid-April. But CrowdTangle data show those accounts are still getting more interactions on their posts than before the pandemic began — in spite of platforms’ policies to limit the spread of misinformation about the virus.
Since January, Facebook and Instagram have been removing posts that "make false claims about cures, treatments, the availability of essential services or the location and severity of the outbreak." The platforms have also banned ads that try to sell face masks, hand sanitizer or other medical supplies.
"To date, we’ve also removed hundreds of thousands of pieces of misinformation that could lead to imminent physical harm," wrote Guy Rosen, Facebook’s vice president of integrity, in an April blog post. They included "claims like drinking bleach cures the virus and theories like physical distancing is ineffective in preventing the disease from spreading."
The platform has also removed coronavirus-related ads. In early April, Facebook spokeswoman Devon Kearns told the Washington Post that "since COVID-19 was declared a public health emergency, Facebook has removed millions of ads and commerce listings for the sale of masks, hand sanitizer, surface disinfecting wipes and COVID-19 test kits."
"While enforcement is not perfect," she added, "we have put several automated detection mechanisms in place to block or remove this material from our platform."
But Facebook has still approved some ads with false or misleading claims about the coronavirus. And some businesses are skirting regulators’ warnings and platforms’ anti-misinformation policies by using coded language or hashtags in their social media posts.
RELATED: 7 ways to avoid misinformation during the coronavirus pandemic
For example, a recent Facebook post from a company called Halosense — to which the FTC sent a warning letter March 30 — claims its salt air purifiers are effective "for all your respiratory ailments." A South African company promoted an intravenous vitamin C product in a recent Instagram post alongside the hashtag #Coronavirus. NewsGuard, a group that tracks online misinformation, has documented several other examples.
Some businesses running ads on Facebook and Instagram are among those targeted by the FTC. A recent ad from a company that sells alkaline supplements promotes supplements as a way to boost your immune system. Another one from a natural health business promotes the use of colloidal silver.
PolitiFact reached out to Facebook to find out how the company is limiting the reach of misinformation about COVID-19 treatments, and whether it’s working with the FTC.
"We have policies against promoting harmful hoaxes related to COVID-19 and exploiting the crisis for financial gain," a Facebook spokesperson told us in an email. "We have already prohibited several of these businesses from using our ads products and are investigating the other ones addressed in these letters."
The spokesperson said Facebook has a dedicated channel for state officials, including attorneys general, to report product listings that they believe violate local laws. Facebook users can also report suspect listings and vendors.
Experts say the persistence of coronavirus-related scams on Facebook and Instagram in spite of those efforts is cause for concern.
Joan Donovan, research director at Harvard University’s Shorenstein Center on Media, Politics and Public Policy, wrote in an April 14 column that the coronavirus pandemic "lays bare the failure to quarantine online scams, hoaxes and lies" on technology platforms.
"Social media is tooled to distribute financial scams, miracle-promising products and fear-mongering conspiracies alongside medical advice, school closures, news and family updates," wrote Donovan, whose team started monitoring misinformation about coronavirus cures in March. "It’s no surprise that damaging guff overwhelms valid recommendations and crucial health information."
PolitiFact partners with Facebook and Instagram to fact-check false and misleading posts. Since January, we’ve fact-checked more than 400 claims about the coronavirus. Once we rate a post as false or misleading, its future reach is limited and users who shared it receive a warning. Facebook reported that, in April alone, the company had flagged about 50 million pieces of coronavirus-related content. (Read more about our partnership with Facebook.)
But with the long wait for a vaccine, regulators aren’t confident that reports of coronavirus-related scams will decrease anytime soon.
"I imagine that if and when we start to have a marked decrease in the number of infections and the number of deaths, things will slow down because you’ll be able to take less advantage of people," said Nessel, the Michigan attorney general. "Until then, I don’t think we’re going to let up for a while."
Now more than ever, it’s important to sort fact from fiction. Please donate to support our mission. Membership.politifact.com
Our Sources
Centers for Disease Control and Prevention, Cases in the U.S., accessed May 19, 2020
Centers for Disease Control and Prevention, How to Protect Yourself & Others, accessed May 27, 2020
Centers for Disease Control and Prevention, Information for Clinicians on Investigational Therapeutics for Patients with COVID-19, April 25, 2020
Centers for Disease Control and Prevention, What to Do If You Are Sick, accessed May 19, 2020
Consumer Reports, "Facebook Approved Ads With Coronavirus Misinformation," April 7, 2020
Consumer Reports, Member Stories: COVID-19 Scams, accessed May 18, 2020
Cornell Legal Information Institute, 21 U.S. Code § 331.Prohibited acts
Cornell Legal Information Institute, 21 U.S. Code § 355.New drugs
Cornell Legal Information Institute, 21 U.S. Code § 352.Misbranded drugs and devices
CrowdTangle, accessed May 20, 2020
Email interview with Eric Weir, May 19, 2020
Email interview with Linda Pavek, owner of CopperTouch, May 5, 2020
Facebook, "An Update on Our Work to Keep People Informed and Limit Misinformation About COVID-19," April 16, 2020
Facebook, "Combating COVID-19 Misinformation Across Our Apps," March 25, 2020
Facebook, "Keeping People Safe and Informed About the Coronavirus," May 4, 2020
Facebook post, May 12, 2020
Google Trends, accessed March 19, 2020
Instagram post, May 13, 2020
International Fact-Checking Network, Fighting the Infodemic: The #CoronaVirusFacts Alliance, accessed May 19, 2020
Interview with Dana Nessel, Michigan attorney general, May 4, 2020
Interview with Joe Potchen, chief of the Michigan Consumer Protection Bureau, May 4, 2020
Interview with Rich Cleland, assistant director of advertising practices at the Bureau of Consumer Protection, April 23, 2020
Interview with Scott Zalkind, owner of Lucky Dog Hot Sauce, May 18 and 20, 2020
Los Angeles Times, "Fake cures, scams, phony medications and price gouging: Predators pounce during coronavirus," April 13, 2020
Los Angeles Times, "Tracking coronavirus in California," May 21, 2020
Mayo Clinic, "My dad takes colloidal silver for his health, but is it safe?"
Michigan Attorney General, "AG's Office Receives New COVID-19 Scam Report, More Than 800 Price-gouging Complaints"
Missouri Attorney General, "AG Schmitt Files Suit Against Jim Bakker for Selling Fake ‘Coronavirus Cure,’" March 10, 2020
Nature, "Social-media companies must flatten the curve of misinformation," April 14, 2020
NBC Bay Area, "Feds Work to Snag Fake COVID-19 Test Kits, Bogus Virus Products," May 5, 2020
NBC News, "States crack down on coronavirus scammers," March 5, 2020
NewsGuard, "Tracking Facebook’s COVID-19 Misinformation ‘Super-spreaders,’" April 23, 2020
New York Attorney General, Bakker Cease and Desist Letter Notification, March 3, 2020
The New York Times, "How the Coronavirus Pandemic Unfolded: a Timeline," May 12, 2020
NPR, "COVID-19 Has Caused A Shortage Of Face Masks. But They're Surprisingly Hard To Make," March 16, 2020
NPR, "Missouri Sues Televangelist Jim Bakker For Selling Fake Coronavirus Cure," March 11, 2020
PolitiFact, "Fact-checking COVID-19 prevention, treatment myths," March 26, 2020
PolitiFact, "How close is a coronavirus vaccine?" May 12, 2020
PolitiFact, "No, drinking bleach will not ward off coronavirus," Jan. 30, 2020
PolitiFact, "Why Trump’s comments on using disinfectants, sunlight to treat COVID-19 are wrong," April 24, 2020
Recode, "Coronavirus scammers are flooding social media with fake cures and tests," April 17, 2020
SiteJabber, Fullerspace, accessed May 19, 2020
U.S. Federal Trade Commission, Federal Trade Commission Act
U.S. Federal Trade Commission, FTC Coronavirus Warning Letters to Companies, accessed May 26, 2020
U.S. Federal Trade Commission, FTC COVID-19 Complaints, May 28, 2020
U.S. Federal Trade Commission, "FTC & FDA: Warnings sent to sellers of scam Coronavirus treatments," March 9, 2020
U.S. Federal Trade Commission, Stipulation to Preliminary Injunction by Defendant Marc Ching, April 27, 2020
U.S. Federal Trade Commission, "‘Thrive’ Supplement Marketer Agrees to Preliminary Order Barring Him from Claiming It Can Treat, Prevent, or Reduce the Risks Associated with COVID-19," April 28, 2020
U.S. Food and Drug Administration, "Fraudulent Coronavirus Disease 2019 (COVID-19) Products," May 27, 2020
The Verge, "Facebook temporarily bans ads for medical face masks to prevent coronavirus exploitation," March 7, 2020
The Washington Post, "A disgraced televangelist promoted an alleged cure to coronavirus. Missouri is now suing him." March 11, 2020
The Washington Post, "The Technology 202: Mask scams and misinformation still present on social media despite tougher policies," April 1, 2020
The White House, "Proclamation on Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID-19) Outbreak," March 13, 2020
Whois.com, accessed May 18, 2020
Wired, "Wellness Influencers Sell False Promises As Health Fears Soar," April 3, 2020