Stand up for the facts!
Our only agenda is to publish the truth so you can be an informed participant in democracy.
We need your help.
I would like to contribute
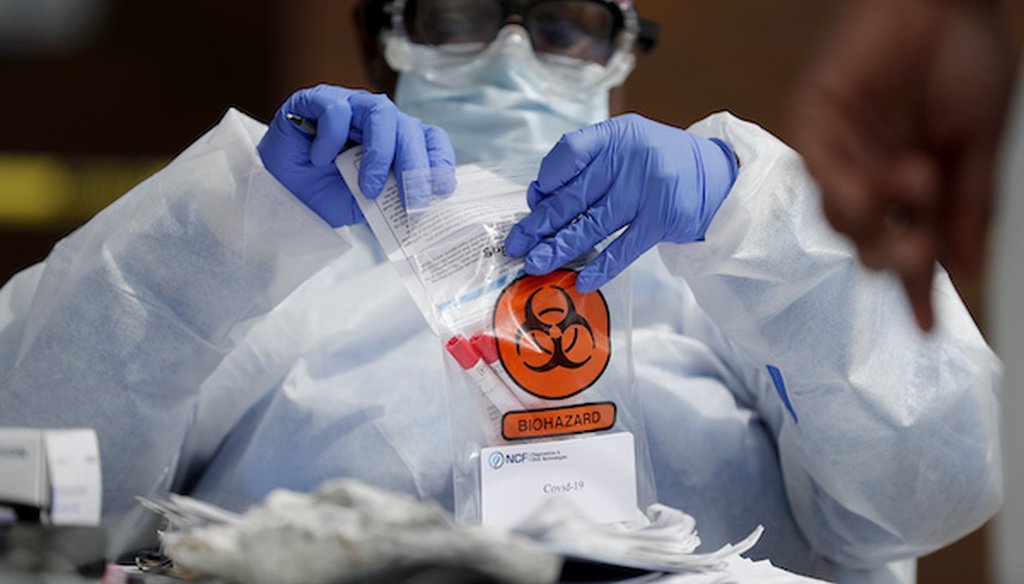
A registered medical assistant handles a nasal swab specimen after it was collected at a drive-thru COVID-19 testing site on April 16, 2020, in St. Louis. (Associated Press)
If Your Time is short
-
Swabs and reagents are used to collect and process samples for coronavirus testing. The sudden worldwide demand for these materials has created a bottleneck and slowed down testing.
-
Government agencies continue to approve and incorporate testing alternatives in an attempt to fix the problem.
President Donald Trump continues to tout the country’s increased testing capabilities in his coronavirus briefings.
He says that there’s a "tremendous amount" of unused testing capacity in states available for governors to tap, and that swabs and reagents — the materials needed to conduct the tests — are "so easy to get."
But several governors say that they aren’t getting enough swabs and reagents to administer large-scale testing. Medical experts stress that more testing is needed before states can safely lift restrictions on people and businesses.
"We could maybe do two or even three times more testing than we are right now," said Michigan Gov. Gretchen Whitmer in an April 21 interview with the Washington Post. "But the fact of the matter is we need those swabs, and we need reagents, so that we can process those tests."
So what, exactly, are these materials that laboratories are clamoring for? Let’s take a look.
Swabs
The swabs that have been required for diagnostic coronavirus tests are not your typical Q-tips.
These swabs have to be long, narrow and flexible enough to reach through the nostril to the nasopharynx, the upper part of the throat that connects with the nasal cavity. The Centers for Disease Control and Prevention says the nasopharyngeal swabs need to be made of synthetic fiber, and cannot be calcium alginate swabs or swabs with wooden shafts, as those may inactivate some viruses and inhibit testing.
While manufacturers have reportedly struggled to keep up with the soaring demand, the CDC recently updated its guidance for health care professionals, and now says other types of swabs — such as a "nasal mid-turbinate (NMT) swab" or a "flocked or spun polyester swab" — can be used in certain situations, including when supplies are low.
The Food and Drug Administration recently greenlit the use of a broader range of swabs, including ones made of polyester, which are expected to be easier to manufacture, and it relaxed other guidelines on storing samples.
In an April 16 announcement, the FDA said that U.S. Cotton, the country’s largest manufacturer of cotton swabs, has developed a "polyester-based Q-tip-type swab" that’s compatible with COVID-19 testing and that the manufacturer plans to produce the new swabs in "large quantities to help meet the needs for coronavirus diagnostic testing."
The agency says that tests with this type of swab, which are less invasive, can be done by patients, allowing for less contact with health care professionals.
RELATED: COVID-19 testing: Where we are now
Reagents
"Reagent" is a broadly used lab term that refers to the key chemical ingredients used in various tests and experiments.
"It’s a generic term," said Dr. Davey Smith, chief of infectious diseases and global public health at University of California-San Diego. "There are reagents for all different kinds of tests. In an extraction kit, they have reagents to extract RNA and nucleic acid, and it’s mostly in a pre-made kit."
For coronavirus testing, a variety of reagents are used in both pure and complex forms, and it’s expensive for individual laboratories to make and process enough to meet the needs in a pandemic.
Smith said U.S. labs could develop or make these reagents on their own, but it’s cheaper to get them from overseas.
"We can make them in the lab. I've made them before," Smith said. "But to any scale, it’s all about the cheaper option."
Which leads us to the shortages.
Smith and other experts say the recent reagent shortages were brought on by the sudden demand for coronavirus diagnostic tests. Labs all over the world are trying to make the same thing, and there aren't enough ingredients to efficiently go around.
"We knew we usually needed X amount, and that’s how much we made," said Smith, who works at the university’s Antiviral Research Center and in his on-campus laboratory. "We didn't see a need for a surge for those reagents. But we’ve had multiple pandemics, and we should have seen it coming. That’s the most frustrating part."
Smith said that the U.S. has ramped up its testing efforts, but still remains far behind where it needs to be to accomplish large-scale testing.
"There’s absolutely more testing," he said. "But we are so far down, it just seems like a drop in the bucket."
Our Sources
CDC, Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19), Updated April 14, 2020
PBS, To speed coronavirus testing, FDA greenlights a new type of nasal swab, April 17, 2020
Food and Drug Administration, Coronavirus (COVID-19) Update: FDA, Gates Foundation, UnitedHealth Group, Quantigen, and U.S. Cotton Collaborate to Address Testing Supply Needs, April 16, 2020
Food and Drug Administration, FAQs on Diagnostic Testing for SARS-CoV-2, Updated April 20, 2020
New York Times, In Scramble for Coronavirus Supplies, Rich Countries Push Poor Aside, April 9, 2020
Radio Free Europe, What's A 'Reagent' And Why Is It Delaying Expanded Coronavirus Testing?, April 18, 2020
Email interview, Kate Fowlie, spokesperson with the Centers for Disease Control and Prevention, April 20, 2020
Phone interview, Dr. Davey Smith chief of Infectious Diseases & Global Public Health as UC San Diego, April 21, 2020












































